Alexander R.A. Anderson, Moffitt Cancer Center & Research Institute, “Steering Cancer Evolution: Harnessing Phenotypic Heterogeneity to Design Better Therapies”
Jump to:
Bio
“Steering Cancer Evolution: Harnessing Phenotypic Heterogeneity to Design Better Therapies”

Alexander R. A. Anderson, PhD is Co-Director of Integrated Mathematical Oncology (IMO) and senior member of Moffitt Cancer Center, Tampa, Florida. His lab is focused on developing organ specific models of tumor initiation and progression that examine the key role of the micro-environment as a selective force in the growth and evolution of cancer. A common theme of these organ specific models is the importance of understanding normal organ form and function particularly in relation to homeostasis. During the last seven years he has closely collaborated with biologists to develop truly integrated models, this has both changed the way biologists do experiments but also the way in which models are developed. Building models that can generate testable hypothesis and utilizing experimental data to parameterize them is a key component of his research.
Dr. Anderson performed his doctoral work on hybrid mathematical models of nematode movement in heterogeneous environments at the Scottish Crop Research Institute in Dundee, UK. His postdoctoral work was on hybrid models of tumor-induced angiogenesis with Prof. Mark Chaplain at Bath University, UK. He moved back to Dundee in 1996 where he worked for the next 12 years on developing mathematical models of many different aspects of tumor progression and treatment, including anti-angiogenesis, radiotherapy, tumor invasion, evolution of aggressive phenotypes and the role of the micro-environment. He is widely recognized as one of only a handful of mathematical oncologists that develop truly integrative models that directly impact upon biological experimentation. His pioneering work using evolutionary hybrid cellular automata models has led to new insights into the role of the tumor micro-environment in driving tumor progression. Due to his belief in the crucial role of mathematical models in cancer research he moved his group to the Moffitt Cancer Center in 2008 to establish the Integrated Mathematical Oncology department.
![]() Click here to view webcast.
Click here to view webcast.
Abstract
“Steering Cancer Evolution: Harnessing Phenotypic Heterogeneity to Design Better Therapies”
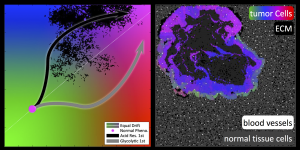
Heterogeneity in cancer is an observed fact, both genetically and phenotypically. Cell-to-cell variation is seen in all aspects of cancer, from early development to invasion and subsequent metastasis. This heterogeneity is also at the heart of why many cancer treatments fail, as it facilitates the emergence of drug resistance. The complex spatial and temporal process by which tumors initiate, grow and evolve is a major focus of the oncology community and one that requires the integration of multiple disciplines. Tumor heterogeneity at the tissue scale is largely due to ecological variations in terms of the tumor habitat driven by spatially heterogeneous vascularity, which is readily observed on cross sectional imaging. Molecular techniques have historically averaged genomic signals from large numbers of cells obtained in a single biopsy site, thus smoothing and potentially hiding underlying spatial variations. The complex dialogue between tumor cells and environment that produces intra- and inter-tumoral heterogeneity is fundamentally governed by Darwinian dynamics. That is, local micro- environmental conditions select phenotypic clones that are best adapted to survive and proliferate and, conversely, the phenotypic properties of the cells affect the environmental properties. While these complex interactions have enormous clinical implications because they promote resistance to therapy, the dynamics are impossible to fully capture via experimentation alone.
Here we present an integrated theoretical/experimental approach to develop dynamical models of the complex multiscale interactions that manifest as temporal and spatial heterogeneity in cancers and ultimately govern tumor response and resistance to therapy. Specifically, we examine the impact of micro-environmental modulation on cancer evolution both in silico, using a hybrid multiscale mathematical model, and in vivo, using three different spontaneous murine cancers. These models allow the tumor to be steered into a less invasive pathway through the application of small but selective biological force. Our long term goal is explicitly translational as we focus our integrated approach on an emerging cancer treatment paradigm that actively harnesses evolutionary dynamics to improve patient outcomes.
![]() Click here to view webcast.
Click here to view webcast.


