Bio
“Arrhythmias, Chance, and Models”

Dr. Raimond L. Winslow is a professor of biomedical engineering at the Johns Hopkins University School of Medicine. He holds an additional appointment in the Whiting School of Engineering at Johns Hopkins, through which he serves as Director of the Institute for Computational Medicine and Director of the Center for Cardiovascular Bioinformatics and Modeling.
Dr. Winslow holds a B.S. in electrical engineering from Worcester Polytechnic Institute and a PhD in biomedical engineering from the Johns Hopkins University. He concluded his training at the Institute for Biomedical Computing and Department of Neurology within Washington University in St. Louis. He joined the faculty of Johns Hopkins in 1991 as an assistant professor, became an associate professor in 1994 and a full professor in 2000.
Dr. Winslow is a fellow of the Biomedical Engineering Society, American Heart Association and American Institute for Medical and Biological Engineering. He serves as Specialty Editor in Chief for the journal Frontiers in Computational Physiology and Medicine, and as a member of the editorial boards of Circulation Research, The Journal of Molecular and Cellular Cardiology, IET Systems Biology and the International Journal of Computational Medicine and Healthcare. He has authored or co-authored over 130 peer-reviewed articles and 12 book chapters, received numerous grants and awards and holds one patent.
 Click here to view webcast.
Click here to view webcast.
Abstract
“Arrhythmias, Chance, and Models”
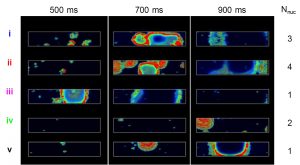
Even in patients with inherited genetic mutations such as channelopathies that pre-dispose them to triggered arrhythmias, arrhythmic events in the heart are highly infrequent, occurring occasionally over the course of billions of heartbeats. However, when the functional effects of these genetic mutations are incorporated into today’s computational models of cardiac cells and tissues, they often predict arrhythmias on nearly every heartbeat. This suggests that there is something fundamentally different about the origins of triggered arrhythmias in real hearts versus models. We consider a certain class of triggered arrhythmias that arise from disturbances in calcium within the cell and hypothesize that these arrhythmias are examples of stochastic rare events. Their stochastic origin results from several interacting factors. The first is governed by the biophysical reality that ion channels controlling myocyte membrane depolarization open and close in a random manner, making the occurrence of a triggered arrhythmic event within a single cell stochastic. The second is governed by the degree of electrical coupling between neighboring myocytes. Due to this electrical coupling, a randomly occurring arrhythmic event in one cell cannot produce a sufficient flow of current into neighboring cells to electrically excite them. Rather, a critical number of nearby cells must undergo these random events in sufficient synchrony that, together, they electrically excite their neighbors. This need for synchrony across a critical number of cells makes triggered arrhythmias in cardiac muscle tissue rare. In this talk we will describe our approach to using stochastic models of cardiac myocytes to study the origins of stochastic arrhythmias in cells and tissue, including approaches for estimating how the probabilities of arrhythmic events depend on underlying cell properties, an approach we call “arrhythmia sensitivity analysis”.
 Click here to view webcast.
Click here to view webcast.