Bio
“Model‐Informed Drug Development: Past, Present, and Future”

Dr. Qi Liu is a Senior Science Advisor in the Office of Clinical Pharmacology (OCP), CDER, FDA. In addition to providing scientific leadership for the regulatory review, she also facilitates regulatory research, innovation and collaboration. She leads OCP’s Innovative Data Analytics program. Before her role as the Senior Science Advisor, Qi worked as a clinical pharmacology team leader and contributed to over 200 NDA/sNDA reviews, 20 BLA/sBLA reviews, and numerous IND reviews to support oncology drug development. She co-authored about 40 manuscripts and presented on many topics at FDA Advisory Committee meetings and scientific conferences. She worked on several working groups for FDA guidance documents and Manual of Policies & Procedures (MAPP) development. Qi was the vice chair of the OCP Biologics Oversight Board. Qi is a Fellow of the American College of Clinical Pharmacology. She is also on the editorial board of Clinical and Translational Science and the American Association of Pharmaceutical Scientists Journal. Qi is interested in the application of clinical pharmacology principles, innovative tools (e.g., modeling/simulation, machine learning, mobile technology), big data and real-world evidence to facilitate drug development and advance precision medicine.
To join live event click here: https://wse.zoom.us/j/98527305681
 Recording will be available here after the event.
Recording will be available here after the event.
Abstract
“Model‐Informed Drug Development: Past, Present, and Future”
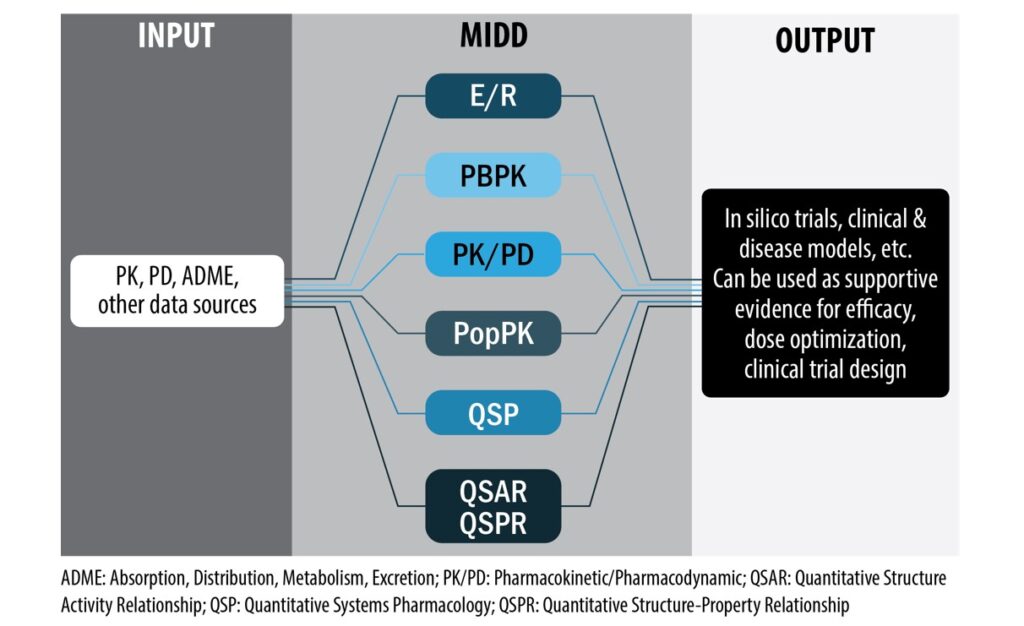
Model‐informed drug development (MIDD) refers to the application of a wide range of quantitative models, which integrate the information about patient characteristics, diseases, and drug effect, to inform drug development and facilitate regulatory decision making. MIDD was formally recognized in Prescription Drug User Fee Act VI, although broadly speaking it has been applied to streamline drug development programs for decades. The regulatory applications of MIDD can be broadly classified into four categories: dose optimization, supportive evidence for efficacy, clinical trial design, and informing policy. In recent years, emerging areas such as more mechanistic models (e.g., quantitative systems pharmacology), neural network models, and real‐world data/evidence are gaining attention, and could possibly expand the scope of MIDD.
Reference: https://ascpt.onlinelibrary.wiley.com/doi/full/10.1002/cpt.1363
 Recording will be available here after the event.
Recording will be available here after the event.